Background
In December, 2020, two mRNA-based COVID-19 vaccines were authorised for use in the USA. We aimed to describe US surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), a passive system, and v-safe, a new active system, during the first 6 months of the US COVID-19 vaccination programme.
Methods
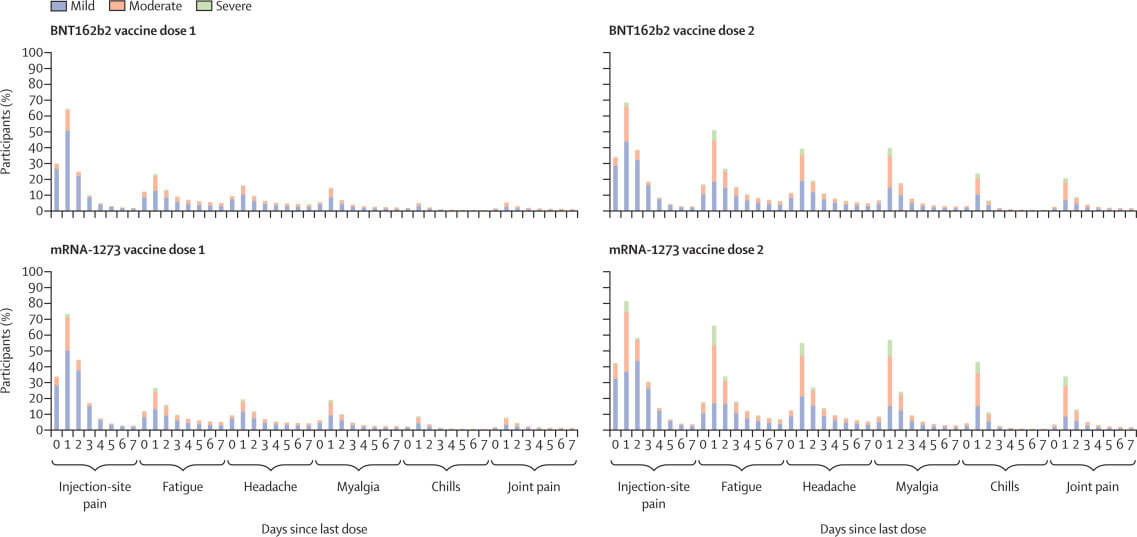
In this observational study, we analysed data reported to VAERS and v-safe during Dec 14, 2020, to June 14, 2021. VAERS reports were categorised as non-serious, serious, or death. Reporting rates were calculated using numbers of COVID-19 doses administered as the denominator. We analysed v-safe survey reports from days 0–7 after vaccination for reactogenicity, severity (mild, moderate, or severe), and health impacts (ie, unable to perform normal daily activities, unable to work, or received care from a medical professional).
Findings
During the study period, 298 792 852 doses of mRNA vaccines were administered in the USA. VAERS processed 340 522 reports: 313 499 (92·1%) were non-serious, 22 527 (6·6%) were serious (non-death), and 4496 (1·3%) were deaths. Over half of 7 914 583 v-safe participants self-reported local and systemic reactogenicity, more frequently after dose two (4 068 447 [71·7%] of 5 674 420 participants for local reactogenicity and 4 018 920 [70·8%] for systemic) than after dose one (4 644 989 [68·6%] of 6 775 515 participants for local reactogenicity and 3 573 429 [52·7%] for systemic). Injection-site pain (4 488 402 [66·2%] of 6 775 515 participants after dose one and 3 890 848 [68·6%] of 5 674 420 participants after dose two), fatigue (2 295 205 [33·9%] participants after dose one and 3 158 299 participants [55·7%] after dose two), and headache (1 831 471 [27·0%] participants after dose one and 2 623 721 [46·2%] participants after dose two) were commonly reported during days 0–7 following vaccination. Reactogenicity was…